
Back Digerman German دی ژرمان Persian Digermane French ジゲルマン Japanese Дигерман Russian Digerman Serbo-Croatian Digerman Serbian 乙锗烷 Chinese
| |

| |
| Names | |
|---|---|
| IUPAC name
Digermane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.159.079 |
PubChem CID
|
|
| |
| |
| Properties | |
| Ge2H6 | |
| Molar mass | 151.328 g/mol |
| Appearance | Colorless gas |
| Density | 1.98 kg/m3[1] |
| Melting point | −109 °C (−164 °F; 164 K) |
| Boiling point | 29 °C (84 °F; 302 K) |
| Insoluble | |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H220, H302, H312, H315, H319, H330, H335 | |
| P210, P260, P261, P264, P270, P271, P280, P284, P301+P312, P302+P352, P304+P340, P305+P351+P338, P310, P312, P320, P321, P322, P330, P332+P313, P337+P313, P362, P363, P377, P381, P403, P403+P233, P405, P501 | |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
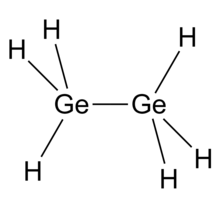
Digermane is an inorganic compound with the chemical formula Ge2H6. One of the few hydrides of germanium, it is a colourless liquid. Its molecular geometry is similar to ethane.[2]
- ^ Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). Boca Raton, FL: CRC Press. pp. 4–61. ISBN 9781498754293.
- ^ Pauling, Linus; Laubengayer, A. W.; Hoard, J. L. (1938). "The Electron Diffraction Study of Digermane and Trigermane". Journal of the American Chemical Society. 60 (7): 1605–1607. doi:10.1021/ja01274a024.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search